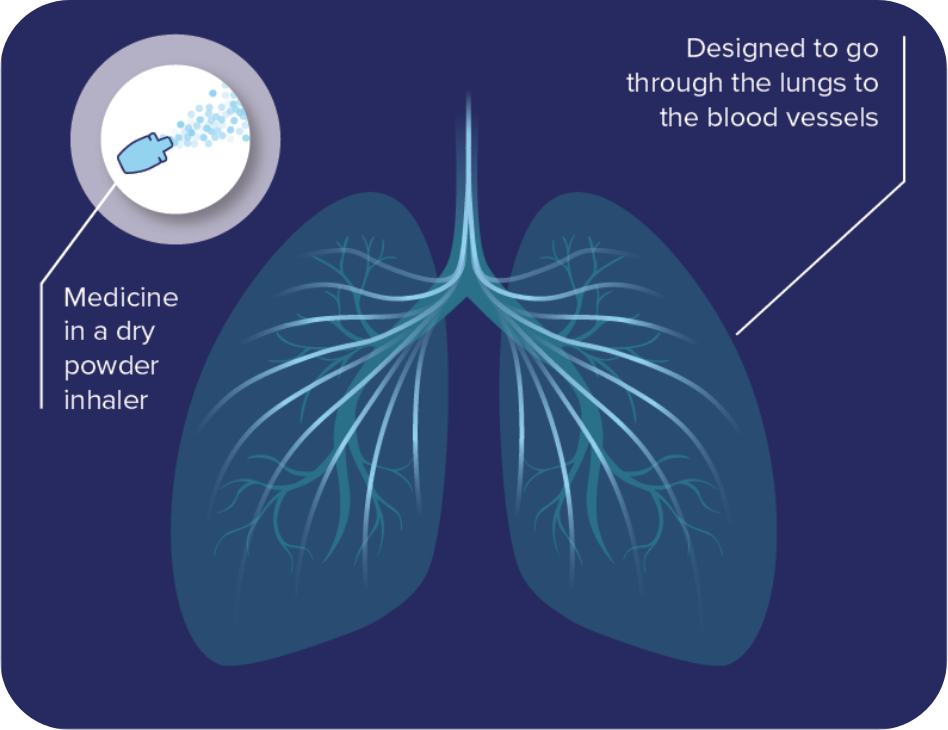
Who can take part?
You or someone you care for may be able to take part if you/they:
- Are 18–75 years of age
- Have been diagnosed with PAH
- Have been receiving stable doses of at least 2 PAH medications for at least the past 90 days
Potential study participants will need to be assessed at a medical center participating in the IMPAHCT trial to confirm whether or not they meet all of the criteria for participation in the trial.